Bioactive Colostrum
Bovine Colostrum is defined as the first milk secreted by the mammary glands after delivery, generally within the first 72 hours. It contains an extraordinary amount of proteins, fat, lactose, vitamins and minerals, representing the first nutritional constituents to the newborn calves.
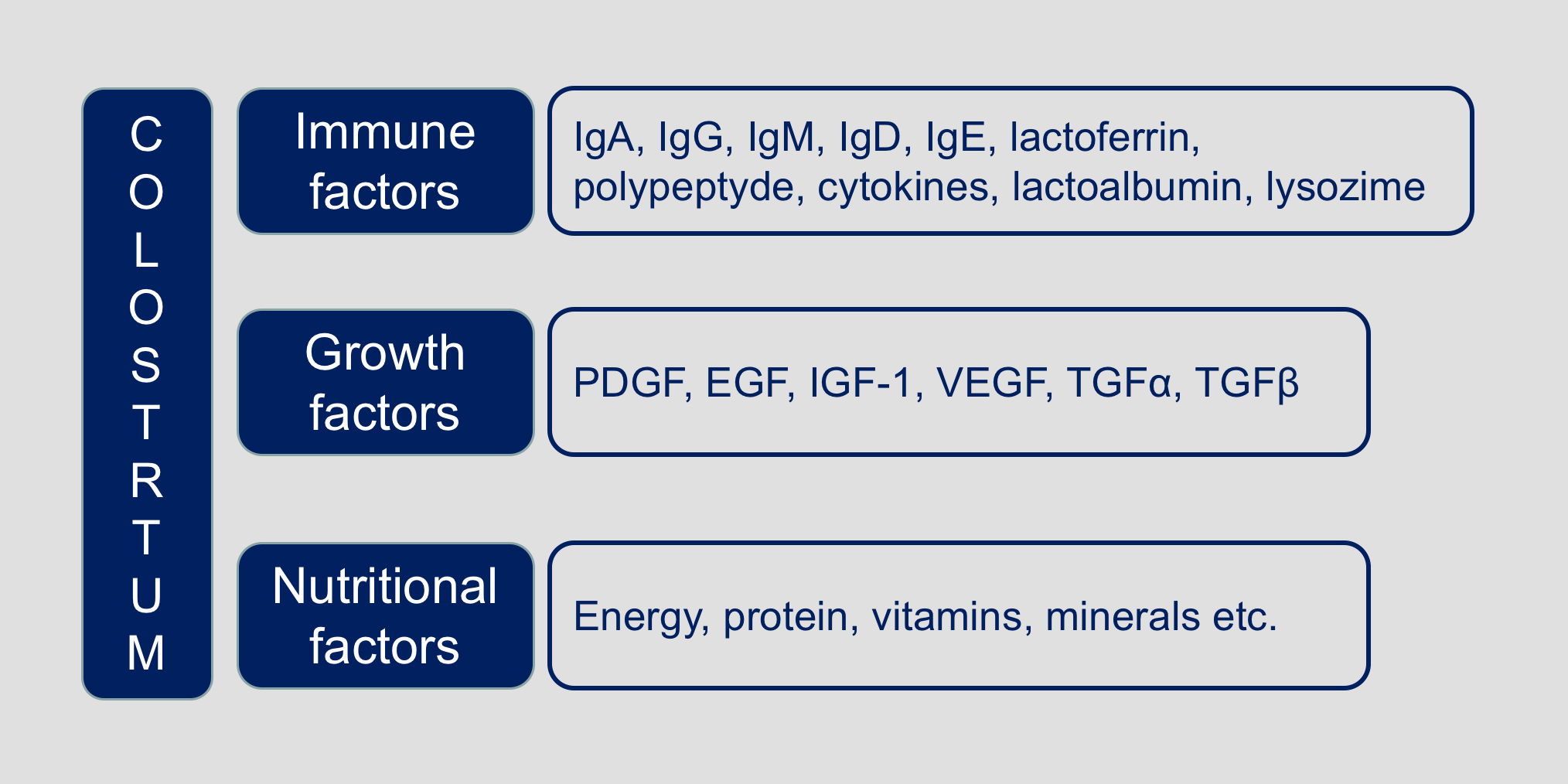
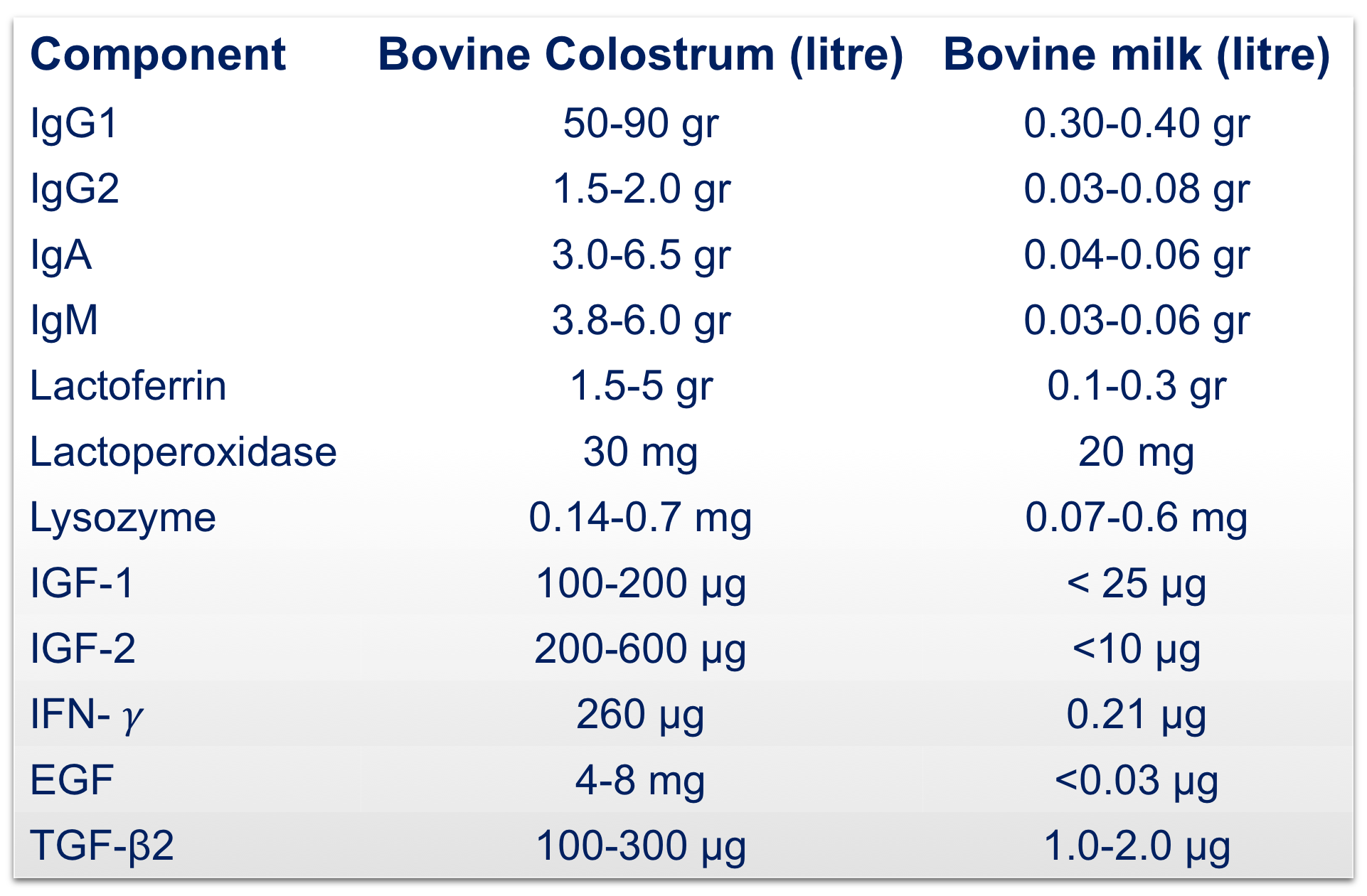
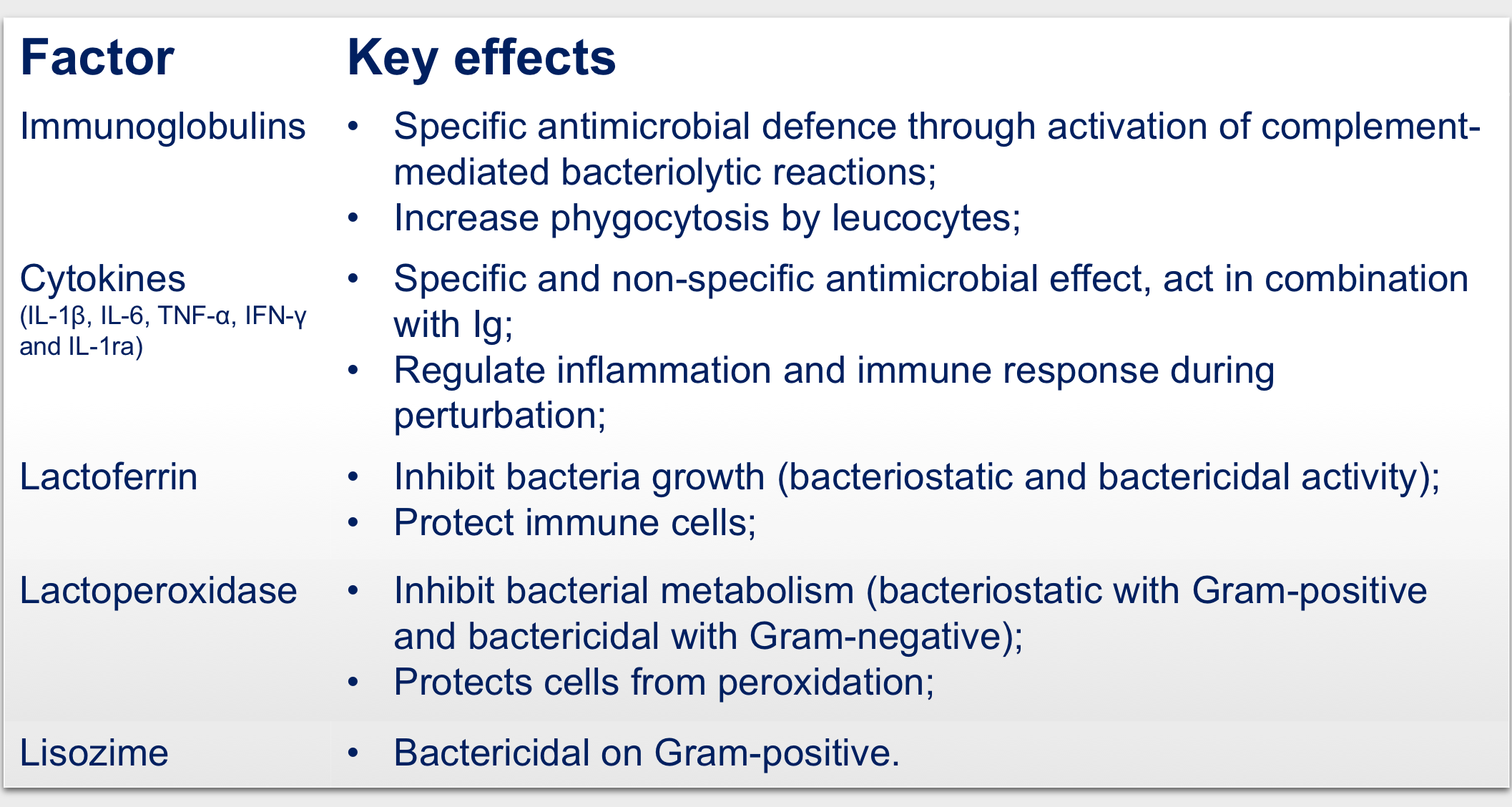
Beyond the nutritional role, colostrum is key for the passive immunity of calves thanks to the presence of immune factors such as lactoferrin, immunoglobulins, lactoperoxidase, cytokines, lactoalbumin and lysozyme, that actively participate in the protection of the neonate.
For the reasons described above colostrum is employed either as food supplement or topical preparation in a variety of conditions and indications.
However, the quality and composition of colostrum may vary substantially and the manufacturing process is critical to determine the final quality.
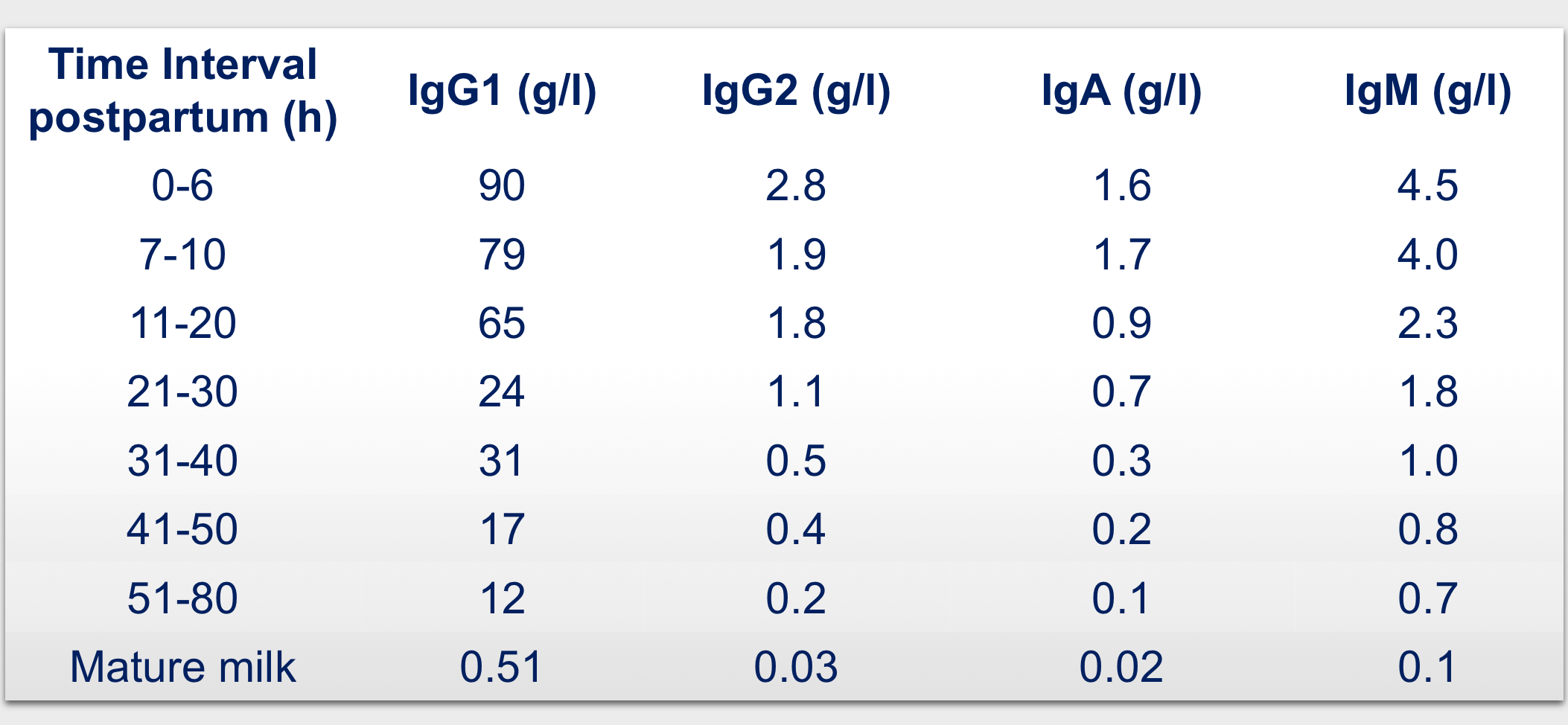
The colostrum employed in the medical devices comes from a novel and patented manufacturing process fully carried out in GMP process that was set-up in order to obtain a safe, bioactive and water soluble natural bovine colostrum. Only milk within 6 hours after parturition is employed in order to maximize the content of actives.
Of note, the procedure utilized to produce the colostrum employed in the medical devices for wound care, preserve most of the bioactive factors in colostrum and these appear to be at the basis of its nutritional, modulatory, and clinical use.
In summary, the employed colostrum is characterized by the following characteristics:
- herd control to minimize the risk of zoonosis and BSE;
- first milking (< 6 hours after delivery) to increase content in actives;
- cold process to preserve actives and avoid unfolding of proteins;
- defatted to reduce oxidation and cytotoxic component;
- casein depleted to reduce immunogenic substances;
- lactose depleted to reduce formation of pro-inflammatory end-glycation products;
- microfiltered to reduce bacterial burden;
- freeze dry to allow conservation.
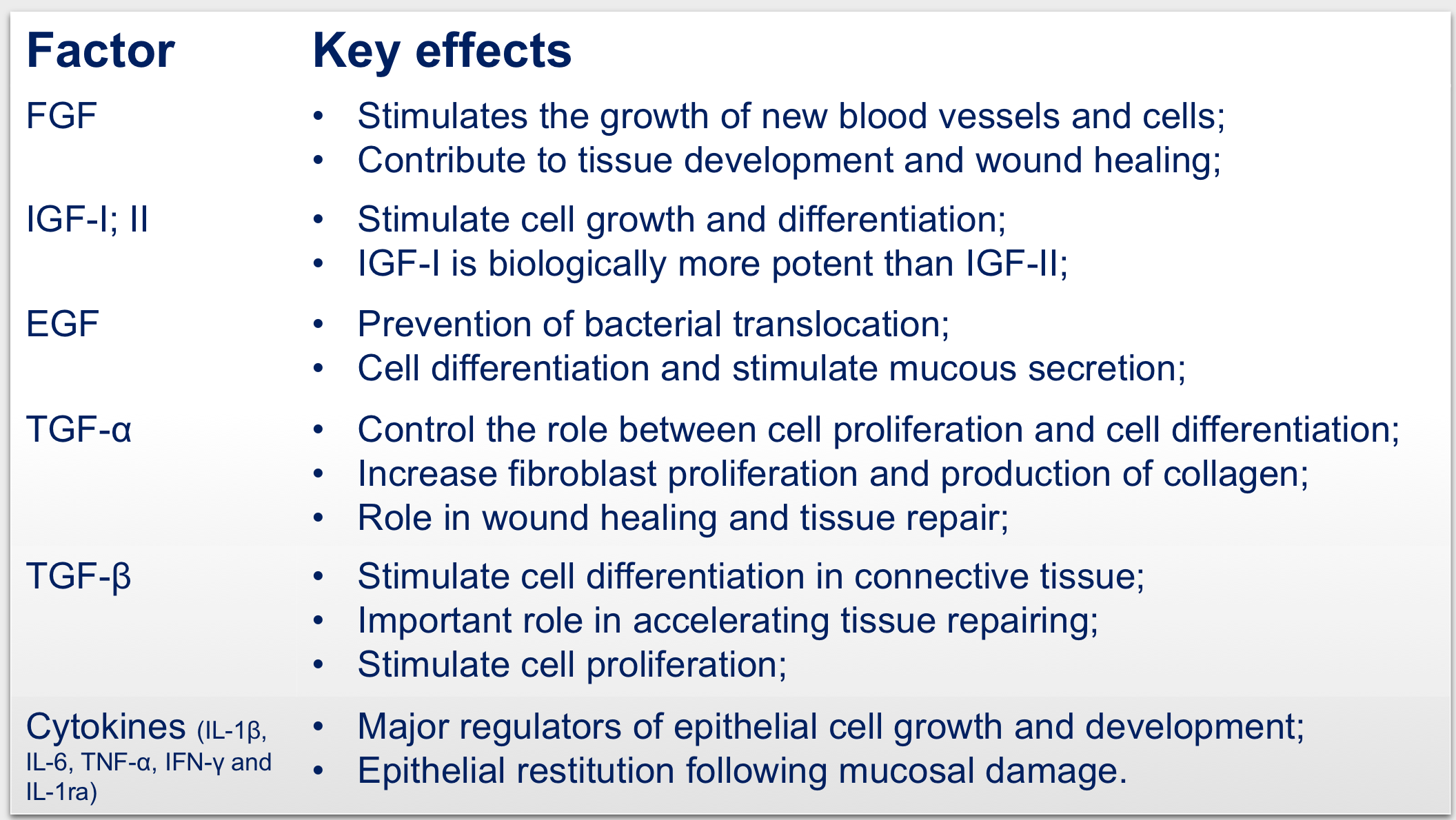
Furthermore, in-depth proteomic analysis of the colostrum employed has identified more than 1’700 proteins and a set of 90 proteins involved in the wound healing process that explain its efficacy in the indicated clinical conditions.
Of note, despite there is a good number of preparations for wound healing on the market there are still unmet medical needs to be addressed.
In fact, current topical preparations act:
- as moisturizer, emollient or
- to limit microbial proliferation or
- as local anaesthetics or
- as physical barrier action or
- to reduce inflammatory process.
Only a few of them though have a repairing effect, and none of them provide a complete action.
Colostrum combines all the above-mentioned properties and in virtue of the presence of growth factors exert a strong repairing action of the damaged tissue.
References:
- Kehoe S.I. et al. J Dairy Sci 90 (9); 4108-4116; 2007.
- Sacerdote P. et al. J Dairy Sci 96 (3); 1745-1754; 2013.
- Godhia & Patel, Curr Res Nutr Food Sci J; Vol. 1(1), 37-47; 2013.
- Boudry C. et al. Biotechnol Agronom Soc et Environ; 12(2); 157-170; 2008.
- Sporn E. et. al. Science, 219 pp. 1329-31; 1983.
- Kshirsagar A. et al Journal of Clinical and Diagnostic Research. Vol-9(4): PC01-PC04; 2015.
- Esmaeili H. et al. RJMS; 21 (125) : 66-74; 2014.
- Patil S.U. et al. RJPBCS 6(5) Page No. 661; 2015.
- Read L.C. et al. J Dev Physiol 7: 135-45; 1985.
- Dignas A.U. et al. Gastroenterol; 105: 1323-32; 1993.
- Marchbank T. et al. Gut; 42 (suppl): A68 (abstr); 1998.
- Jin Y. et al. J Protein Chem; 10: 565-75; 1991.
- Daughaday W.H. et al. Endocr Rev; 10: 68-91; 1989.
- Lund P.K. et al. Clin Gastroenterol; 10: 83-96; 1996.
- Baxter R.C. et al. J Clin Endocrinol Metab; 58: 955-9; 1994.